|
The alcohols are a homologous series with the hydroxyl -OH functional group attached to one of the carbon atoms. They have the general formula CnH2n+1OH. Structural isomerism is possible, as the position of the OH group can vary. Naming follows the usual patterns, with the carbon chain numbered to give the hydroxyl group the lowest possible number. Alkyl groups are then numbered accordingly.
Alcohols exhibit two forms of intermolecular bonding: permanent dipole-dipole attractions between the polar ľOH groups of neighbouring molecules, and temporary dipole-dipole attractions which form between the alkyl groups. In the smaller alcohols, the permanent dipole-dipole attractions dominate, and for this reason, the first three alcohols are all soluble in water (water is polar, and like dissolves like). The 4th, 5th and 6th alcohols are also slightly soluble. In the larger alcohols, it is the longer alkyl chain that is responsible for most of the physical properties (temporary dipole-dipole attractions become stronger with increasing molar mass), and so the larger alcohols are insoluble. Melting and boiling points of the alcohols are much higher than the corresponding alkanes, due to their stronger permanent dipole-dipole bonding.
In addition to their structural isomerism, the alcohols can also exhibit a new form of isomerism - optical isomerism. In order to exhibit such isomerism, the alcohol must contain a carbon bonded to four different atoms or groups of atoms, known as an asymmetric carbon. A molecule containing an assymetric carbon is known as a chiral molecule, and the two forms of a chiral molecule are the optical isomers. These two are mirror images of one another, and cannot be superimposed. While the physical and chemical properties of optical isomers are very similar, they can be distinguished, as "the two isomers will rotate the plane of polarised light in different directions".
An optical isomer as seen from (1) the side, then (2) the top, and finally its mirror image (3) to show that they cannot be superimposed, much like a right hand cannot be superimposed onto a left hand.
Alcohols are classified based on the number of other carbons bonded to the OH group carbon.
Generally, a 1 in the name will indicate a primary alcohol e.g. butan-1-ol, a 2 or 3 will point out a secondary alcohol, like pentan-2-ol, and a repeating number often shows a tertiary alcohol, where an alkyl group must also be attached to the hydroxyl carbon e.g. 2-methylbutan-2-ol.
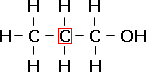
propan-1-ol, a primary alcohol. |
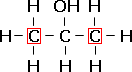
propan-2-ol, a secondary alcohol. |
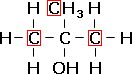
methylpropan-2-ol, a teriary alcohol. |
REACTIONS
(1) Like alkenes, alcohols undergo combustion to form heat, water and carbon dioxide. Carbon monoxide or carbon may also form. As they produce small amounts of pollution when burnt, use of alcohols as an alternative fuel source is being investigated.
(2) By using oxidising agents such as acidified dichromate or permanganate solution, primary and secondary alcohols can both be oxidised. When acidified dichromate is reduced, it changes colour from orange to blue green due to the formation of chromium ions in solution.
Cr2O72- + 14H+ + 6e- ==> 2Cr3+ + 7H2O
When using permanganate as the oxidant, the colour change is from purple to colourless, according to the reaction equation:
MnO4- + 8H+ + 5e- ==> Mn2+ + 4H2O
Primary alcohols are oxidised to aldehydes, or to carboxylic acids under reflux:
C2H5OH ==> CH3CHO + 2H+ + 2e-
C2H5OH + H2O ==> CH3COOH + 4H+ + 4e-
Secondary alcohols are oxidised to form ketones:
CH3CHOHCH3 ==> CH3OCH3 + 2H+ + 2e-
Tertiary alcohols cannot be readily oxidised.
(3) Alcohols are also used in the production of chloroalkanes, by chlorination. There are 3 common reagents used:
| [A] Phosphorous trichloride | PCl3 + 3CH3OH ==> 3CH3Cl + H3PO3 |
| [B] Phosphorous pentachloride | PCl5 + C2H5OH ==> C2H5Cl + POCl3 + HCl |
| [C] Thionyl chloride | C2H5OH + SOCl2 ==> C2H5Cl + SO2 + HCl |
(4) The Lucas Test enables us to differentiate between primary, secondary and tertiary alcohols. Lucas Reagent is a mixture of anhydrous ZnCl2 and concentrated hydrochloric acid. When added to an alcohol, insoluble chloroalkanes are formed (the ZnCl2 is a catalyst) as a cloudy white precipitate. The speed of the reaction indicates which type of alcohol is present.
Primary alcohols react slowest with Lucas reagent, and the white precipitate will take in excess of 15 minutes to form, if it is to form at all. Secondary alcohols react faster, and the white ppt. will develop in 5-15 minutes. The tertiary alcohols react comparatively quickly, cloudiness developing within 1-2 minutes. Thus, the three classes of alcohols can be differentiated experimentally.
(5) Alcohols can be used to produce alkenes by dehydration. Using a reagent of concentrated H2SO4 at a temperature of 170░C, the alcohol undergoes the following reaction:
Using ethanol as an example: C2H5OH ==> C2H4 + H2O
Note that [Markovnikov's rule] for addition reactions applies here.
|

