|
[Introduction] [Formulae] [Naming]
Organic Chemistry is the study of carbon compounds. Usually, organic molecules also contain hydrogen, and sometimes include other elements like nitrogen and oxygen. The carbon atom has 4 valence electrons, and can form four covalent bonds. For this reason, there is an enormous number of organic compounds possible. The covalent bonds can exist as single, double or triple bonds, both to carbon or other elements, and can form straight chain, branched, or cyclic structures. Organic compounds have a wide variety of uses - many are used as fuels, solvents and plastics.

There are five main types of formulae that can be used to describe an organic molecule:
| The simplest is the empirical formula, which merely gives a ratio of atoms within the particular element. | 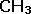 |
| The molecular formula is slightly more enlightening, giving exact numbers of each atom present. | 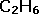 |
| The constitutional formula gives an arrangement of atoms within a molecule | 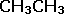 |
| The structural formula shows the arrangement of atoms, in 2-D. The structural formula also indicates bonding. | 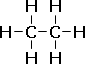 |
| Finally, the 3-D formula shows the actual shape of the molecule, in 3D. Bonds that go into the page are shown by dashes, bonds coming out are shown by triangles. | 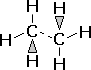 |
In general, the most useful formulae are the constitutional, 3D and structural formulae. Without further information, naming is only possible given one of these three formulae.
All organic compounds have specific names, assigned using specific conventions. From these names, the organic structure can be determined, and vice versa. All organic compounds make use of a common set of prefixes to describe the parent or main chain (defined by the longest possible carbon chain), based on the number of carbon atoms
| Number of Carbon Atoms in Parent Chain | Prefix |
| 1 | Meth- |
| 2 | Eth- |
| 3 | Prop- |
| 4 | But- |
| 5 | Pent- |
| 6 | Hex- |
| 7 | Hept- |
| 8 | Oct- |
| 9 | Non- |
| 10 | Dec- |
The suffix then indicates the type of organic compound. For instance, a carboxylic acid will have the suffix "-oic acid" and the functional group -COOH. Multiple functional groups are indicated by adding on a "di" for two, "tri" for three or "tetra" for four before the appropriate functional group - an organic compound with two COOH groups is a Dioic Acid.
The presence of double or single bonds will also change the name. For example, hexanoic acid has a single bond, but hexenoic acid has a double bond, and hexynoic acid has a triple bond.
Side chains have a similar naming pattern to parent chains e.g. -CH3 is methyl, -CH2CH3 is ethyl etc. Multiple alkyl groups are indicated by adding on a "di" for two, "tri" for three or "tetra" for four before the prefix. The positions of these alkyl groups are then indicated in the name by using numbers, which identify which parent chain carbon the group is attached to. Numbers are separated by commas, and numbers and letters must be separated by a hyphen. For example, 2,4-dimethylhexandioic acid.
Converting Names to Structural and Constitutional Formula
When attempting to convert a molecule's name into its structural or constitutional formula, it is often helpful to break the name up into sections. Generally, you start with the main chain, add the functional group second, and add any side-chains (and hydrogens, oxygens etc.) last.
Example: 2,4-dimethylhexandioic acid, molecular formula C8H16O4
| Step One: The main chain has a prefix hexan- and so has 6 carbon atoms, each with single bonds. | 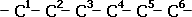 |
| Step Two: -dioic means there are two carboxylic acid functional groups. By convention, they always go on the ends of the carbon chains, so add a 'OOH' to carbon #1 and carbon #6. | 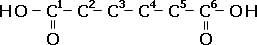 |
| Step Three: 2,4-dimethyl means there are two additional methyl groups. Moreover, they are on carbons #2 and #4. | 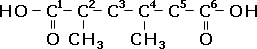 |
| Step Four: Since there is nothing else in the name to cover, add hydrogen atoms until each carbon atom has four bonds. | 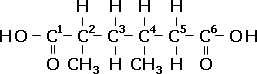 |
Written as a constitutional formula, the structure would be: COOH-CH(CH3)-CH2-CH(CH3)-CH2-COOH
Any key points needed for drawing structure from a constitutional formula can be identified here, by analysing the constitutional formula, given the structure. Any alkyl groups within the molecule in brackets will be present as side chains, attached to the preceding carbon of the parent chain (The CH(CH3) part, for example). However, if a group is in brackets, and has one less hydrogen than its corresponding alkyl group, it is part of the main chain.
Note how the carboxyl group is said to be attached to the first carbon atom, rather than the second - if it was the second, the structure would be CH3-CH(COOH)-CH2-CH(CH3)-CH2-COOH, which is incorrect.
|

