|

Aldehydes and Ketones both contain a C=O group known as a carbonyl group. For this reason, they are known as carbonyl compounds.
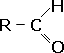
| The aldehydes have the systematic name alkanal. They are an homologous series containing a carbonyl group on one of the end carbons, forming a -CHO functional group. They have the general formula CnH2n+1CHO. Numbering follows the usual rules (see [Introduction - Naming]) with the suffix -al used to indicate the presence of the -CHO functional group.
|
aldehydes |
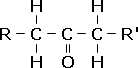
| Unlike the aldehydes, in the ketones the carbonyl group is located somewhere in the main carbon chain, hence the first ketone member is propanone. They have the general formula CnH2n+1COCnH2n+1. The ketones are named with the suffix -one, and a number indicating the carbon to which the oxygen is attached e.g. pentan-2-one, which has the formula CH3COCH2CH2CH3.
|
ketones |
As the carbonyl group is polar, both the aldehydes and ketones have permanent dipole-dipole attractions between molecules. The polarity is fairly strong, and so intermolecular bonding is also reasonably strong, most aldehydes and ketones existing as volatile liquids. They have definitive odours, aldehydes smelling unpleasant and pungent, whereas ketones usually have pleasant sweet smells. Smaller aldehydes and ketones are soluble in water due to their polarity, with aldehydes and ketones smaller than about 5 carbons long showing some solubility.
REACTIONS
(1) As has already been covered in the [alcohols] section of this web-page, aldehydes are prepared through the oxidation of primary alcohols. Common oxidising agents used are acidified dichromate and permanganate solutions.
The aldehyde may also be oxidised to form a carboxylic acid. To prevent this from happening, the aldehyde produced is removed through gentle heating of the reaction vessel, and then recondensed into a separate flask. This method is effective for many of the aldehydes, depending on their boiling point compared to the boiling point of the alcohol.
The ketones are prepared by oxidation of secondary alcohols. This reaction is performed under reflux, to increase reaction rate and ensure that all of the alcohol is oxidised.
(2) Aldehydes are more reactive than ketones; they are more readily oxidised. This property is the basis of identification tests for aldehydes. Aldehydes will be readily oxidised to carboxylic acids, where ketones will not. This reveals a variety of methods to distinguish between them. Acidified dichromate or permanaganate solutions will both react with aldehydes, resulting in colour changes (from orange to green, and purple to colourless respectively) but will have no reaction with ketones.
(3) Another possible test uses Tollen's reagent. Tollen's reagent contains
the diamminesilver (I) complex ion:
[Ag(NH3)2]+
Aldehydes cause the reduction of the silver ions to silver metal, which appears as a 'silver mirror' inside the test tube. Ketones do not have any reaction with Tollen's reagent. The reaction equation for ethanal is:
CH3CHO + 2[Ag(NH3)2]+ + H2O + 2H+==> CH3COOH + 2Ag + 4NH4+
(4) Finally, aldehydes can be tested for using Benedict's solution. Benedict's solution contains aqueous copper (II) ions in
alkaline conditions. These copper ions exist as a complex with citrate ions, to prevent the precipitation of copper hydroxide. When it is warmed with an aldehyde, Cu2O
forms, resulting a reddish brown precipitate:
CH3CHO + 4OH- + 2Cu2+ ==> CH3COOH + Cu2O + 2H2O
Fehling's solution behaves in a similar manner, only the Cu2+ ions are complexed with tartrate. Ketones will not react with either, as ketones cannot reduce metal ions.
|

