|
[Carboxylic Acids] [Acid Chlorides] [Esters] [Amides] [Amino Acids]
Carboxylic Acids have a functional group of -COOH. They are formed through the oxidation of Alcohols and Aldehydes -
Alcohol
R-CH2-OH |
==> |
Aldehyde
R-CHO |
==> |
Carboxylic Acid
R-COOH |
Due to the highly electronegative nature of oxygen, the C=O bond is very polar. Hence, the positive carbon attracts negatively charged molecules called "nucleophiles" which combine with carboxylic acids to form compounds such as Esters, Amides and Acid Chlorides.
Acid chlorides are made from carboxylic acids with a chlorine atom replacing the hydroxyl group of the COOH group. The systematic name for acid chlorides is alkanoyl chlorides and they have a general formula of CnH2n+1COCl.
The naming and positioning of any side chains is done using the carbon of the COCl as the first carbon atom in the parent chain. The functional group -COCl is given the prefix -oyl chloride.
Acid chlorides form no hydrogen bonds between molecules and thus generally have low melting and boiling points as a result. They are usually fuming, pungent liquids.
REACTIONS
Like carboxylic acids, the strong positive carbon atom results in acid chlorides being highly reactive and susceptible to attacks from nucleophiles, of which some are listed below:
(1) Acid chlorides are formed by the chlorination of carboxylic acids. Two common chlorinating agents are used - PCl5 and SOCl2.
(2) Acid chlorides violently react with water forming a carboxylic acid and hydrogen chloride:
CH3COCl + H2O ==> CH3COOH + HCl
(3) Hydrolysis - Acid chlorides react easily with ammonia forming amides and ammonium chloride:
CH3COCl + 2NH3 ==> CH3CONH2 + NH4Cl
(4) Acid chlorides readily react with primary aminoalkanes to form secondary amides and an ammonium salt:
CH3COCl + 2CH3NH2 ==> CH3CONHCH3 + CH3NH3Cl
(5) Finally, acid chlorides react with alcohols forming an ester and hydrogen chloride:
CH3COCl + C2H5OH ==> CH3COOC2H5 + HCl
Esters are formed from both carboxylic acids and alcohols and are distinguished by the COO- functional group being attached to two parent alkyl chains.
Esters are named according to the alcohol and carboxylic acid which combined to form the ester; for example, ethanol and propanoic acid will react to form ethyl propanoate
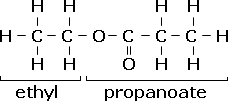 The carbon attached to two oxygens comes from the carboxylic acid - hence that chain will have the -oate suffix. The other parent chain is named accordingly using standard meth-, eth- etc prefixes.
The carbon attached to two oxygens comes from the carboxylic acid - hence that chain will have the -oate suffix. The other parent chain is named accordingly using standard meth-, eth- etc prefixes.
Esters are usually oily, colourless liquids with characteristic smells of fruits or flowers. Compared to their corresponding alcohol or carboxylic acid they have lower boiling points as esters do not form hydrogen bonds between their molecules.
REACTIONS
(1) Formation - "Esterfication" reactions are condensation reactions between an alcohol and carboxylic acid or an acid chloride to form esters. The reaction is usually performed under reflux apparatus and a catalyst of concentrated sulfuric acid is usually used to speed up the rate of reaction. Formation of ethyl propanoate is outlined below:
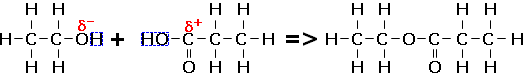 A water molecule is a by-product of the reaction. Note that the oxygen bonded to two carbons comes from the alcohol - this is due to the negative charge being attracted to the positive carbon on the carboxylic acid.
A water molecule is a by-product of the reaction. Note that the oxygen bonded to two carbons comes from the alcohol - this is due to the negative charge being attracted to the positive carbon on the carboxylic acid.
The principle of the reaction is exactly the same when using an acid chloride instead of a carboxylic acid. Hydrogen chloride is a by-product instead of water. This reaction will not need a catalyst due to the extremely reactive nature of the acid chloride.
(2) Hydrolysis - The reversal of all the above reactions is possible, this is known as the hydrolysis of esters. This can be performed under different conditions, which will produce different products:
- Acidic conditions will result in a carboxylic acid and alcohol being formed.
- Alkaline conditions will result in a carboxylate ion or salt and alcohol being formed.
(3) Saponification - Saponification, or soap making, is the hydrolysis of triesters of glycerol under alkaline conditions.
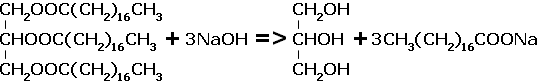 The products are sodium sterate, and glycerol, the systematic name is propan-1,2,3-triol.
The products are sodium sterate, and glycerol, the systematic name is propan-1,2,3-triol.
The systematic name for amides is "alkanamides" and contain the functional group –CON–
Classification and naming is exactly the same as the aminoalkanes, except the suffix -amide is used instead of -amine.
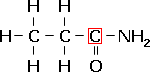
propanamide, a primary amide. |
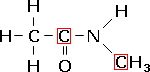
N-methylethanamide, a secondary amide. |
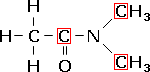
N,N-dimethylethanamide,
a teriary amide. |
With the exception of methanamide, all amides are white, crystalline solids. All amides are soluble in organic solvents and are usually odourless if pure. Amides with a low molecular mass are also soluble in water. All have high melting points as the oxygen in the carbonyl group is able to hydrogen bond with a hydrogen atom on a surrounding amide molecule.
REACTIONS
(1) Formation - Primary Amides can be made three different ways:
- Heating a carboxylate salt to form an amide and water
- Reacting an ester with concentrated aqueous ammonia to form an amide and alcohol
- Reacting an acid chloride with concentrated aqueous ammonia to form an amide and ammonium chloride.
Secondary amides can be made by reacting acid chlorides with primary amines.
Tertiary amides can be made by reacting acid chlorides with secondary amines.
(2) Hydrolysis - Like esters, amides can be hydrolysed simply by heating under either acidic or basic conditions:
- Acidic conditions will form a carboxylic acid and ammonium ions.
- Alkaline conditions will form the carboxylate ion and ammonia.
The amino acids contain two functional groups- the amino group, NH2 and the carboxylic acid group COOH. The general formula for an alpha amino acid is displayed below:
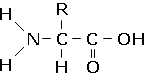 All naturally occuring amino acids are alpha amino acids. Beta-amino acids may also be synthesised, in which the amino group is attached to the third carbon atom of the chain, rather than the second. The simplest of the aminoacids, glycine, has a hydrogen atom as the R group on the second carbon. Therefore, it cannot exhibit optical isomerism (there is no asymmetric carbon atom). However, all the other amino acids will form optical isomers. In nature, only one of each optical isomer can be utilised by living organisms.
All naturally occuring amino acids are alpha amino acids. Beta-amino acids may also be synthesised, in which the amino group is attached to the third carbon atom of the chain, rather than the second. The simplest of the aminoacids, glycine, has a hydrogen atom as the R group on the second carbon. Therefore, it cannot exhibit optical isomerism (there is no asymmetric carbon atom). However, all the other amino acids will form optical isomers. In nature, only one of each optical isomer can be utilised by living organisms.
| 
