|
[Alkanes] [Haloalkanes] [Aminoalkanes]
Alkanes are the simplest of all organic compounds. They are known as hydrocarbons, consisting solely of carbon and hydrogen atoms. They can also be classified as a homologous series, whereby each member differs from the previous one by a –CH2 unit. The alkanes are a family of saturated hydrocarbons, meaning that all carbon to hydrogen bonds are single. They have the general formula CnH2n+2. Alkanes follow the same naming pattern as was mentioned in the introduction to organic chemistry: CH4 is methane, C2H6 is ethane, and so on.
Alkanes can exhibit structural isomerism through branched chain alkanes. A structural isomer is a compound that has the same molecular formula, but a different arrangement of atoms. Two such isomers are hexane and 3-methylpentane. While both have the molecular formula C6H14, their structures differ.
A closely related family are the cycloalkanes. These, too, are a family of saturated hydrocarbons. However, they exist as ring structures, and have the general formula CnH2n, having two hydrogens less than their straight chain relatives. All hydrocarbon chains with 3 or more carbons can exist in a cyclic state. Naming is the same as for their straight chain equivalents, but with the prefix "cyclo". e.g. cyclopentane, 2-methylcyclobutane.
Alkanes consist of non-polar covalent bonds, and all the valence electrons are fixed within bonds. Therefore, alkanes are insoluble in water (water is polar), and they are non-conductors (there are no free electrons to carry the charge under the influence of an applied electric field). As with most non-polar molecules, melting point and boiling point values increase with increasing molar mass. Methane, ethane, propane and butane occur as gases at room temperature. Pentane to hexadecane (16 carbon atoms long) are oily liquids, and larger alkanes are found as waxy solids.

The haloalkanes are "an homologous series of organic compounds, containing a halide ion in place of one or more hydrogen atoms". The general formula is CnH2n+1X, where X represents a halide ion, whether it be chloride, bromide, iodide, etc. They are formed either by halogenation of alcohols, from alkanes reacting with halogens, or by hydrohalogenation of alkenes.
The haloalkanes, as their name suggests, are named like the alkanes, with the chloro- or bromo- prefix to indicate which type of halide ion is attached. As per usual, the halide ion must be present on the parent chain, and the chain has to be numbered to give the carbon with the halide attached the lowest possible number. Naming of alkyl side chains is also the same as for the alcohols. In fact, in most ways the naming of chloroalkanes is identical to that of the alcohols. Even classification as primary, secondary or tertiary follows the same procedures (See [Alcohols] for more information).
Bonding in haloalkanes involves permanent dipole-dipole attractions. Only chloromethane, bromomethane and chloroethane exist in a gaseous state at room temperature, most of the others with fewer than 6 carbons being liquids. They are only sparingly soluble, the polarity of the C-Cl bond being slight in comparison to the strong polarity of water. They are usually more soluble in non-polar solvents.
Often, chloroalkanes can be used as solvents. CCl4 is used in drycleaning, CH3CCl3 is a component of correction fluid, and CH2Cl2 is an industrial solvent. Chloroalkanes can also undergo polymerisation, and are used in the production of PVC and teflon.
REACTIONS
Chemically, most of the reactions that involve haloalkanes are substitution or elimination reactions. These reactions occur as the carbon atom attracts nucleophiles such as OH- or NH3 due to the highly electronegative chlorine atom causing a strong polar bond. These groups have a lone pair of electrons, and so will bond with the carbon, replacing the halide ion.
(1) When reacted with dilute KOH, the hydroxide group will substitute the halogen, forming an alcohol.
CH3CH2X + OH- ==> CH3CH2OH + X-
(2) With alcoholic ammonia at high temperatures, it is the –NH2 amino group that replaces the halide, according to the equation:
CH3CH2X + 2NH3 ==> CH3CH2NH2 + NH4X
The product is an aminoalkane. This aminoalkane can then react further with the chloroalkane:
CH3CH2NH2 + CH3CH2Cl ==> CH3CH2NHCH2CH3 + HCl
Thus, a primary aminoalkane reacts to form a secondary aminoalkane.
(3) In elimination reactions, the haloalkane forms an alkene by removal of the halide atom using alcoholic KOH or NaOH. Water and a salt are the other products. In the case of chloroethane and KOH:
CH3CH2Cl + KOH ==> CH2CH2 + KCl + H2O
Tertiary haloalkanes will most readily undergo elimination, followed by secondary and then primary haloalkanes. In these reactions, [Markovnikov's rule] applies – The 'poor' get 'poorer'.
CH3CHClCH2CH3 ==> CH3CHCHCH3 + HCl
The alkanamines, or aminoalkanes, have a nitrogen atom attached in the organic molecule. This is indicated by the either an amino prefix, or a amine suffix (eg, ethanamide and aminoethane are different names for the same molecule). They are often used in anaesthetics, drugs and the like, and can be produced from haloalkanes by the addition of alcoholic NH3. Aminoalkanes are classified based on the number of carbons attached to the amine group:
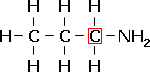
1-propanamine, a primary amine. |
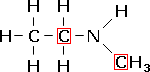
N-methylethanamine, a secondary amine. |
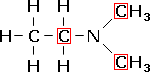
N,N-dimethylethanamine,
a teriary amine. |
The letter 'N' is appended to the beginning to show that the alkyl group is attached to the nitrogen atom, not the main carbon chain. Di and tri indicate multiple alkyl groups, and then naming is the same as for the other organic compounds.
e.g. CH3CH(NH2)CH(NH2)CH3
The main chain has four carbons. Therefore, it is a butane. There are 2 NH2 groups, and so will have the prefix diamino. As these groups are on the second and third carbons, the final name is 2,3-diaminobutane. |
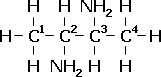 |
Aminoalkanes exhibit hydrogen bonding, as well as the other weaker Van der Waals forces. For this reason, the low molar mass aminoalkanes have relatively high melting and boiling points. Both aminomethane and aminoethane are gases, aminopropane and aminobutane exist at room temperatures as fishy smelling volatile liquids, and the larger aminoalkanes are solids. For the small aminoalkanes, hydrogen bonding dominates, and for this reason, the low molar mass aminoalkanes are soluble in water (water is very polar, and like dissolves like). Solubility decreases as aminoalkanes become larger, as the dipole properties of the long alkyl chain begin to govern.
REACTIONS
Due to the amine group, and the lone pair of electrons attached to the nitrogen, aminoalkanes behave much like ammonia in reactions, one of the hydrogens simply being replaced by an alkyl chain. To that end, aminoalkanes react in essentially the same manner as ammonia. They can act as proton acceptors, as nucleophiles, and as ligands (small groups with a lone pair of electrons that can bond to transition metals to form complex ions). From here on, aminoethane will be used to demonstrate the reaction properties of aminoalkanes.
(1) Acid/Base reactions: With water, the aminoalkanes act as a base, accepting a proton and increasing the pH.
C2H5NH2 + H2O ==> C2H5NH3+ + OH-
Similarly, with acid, the aminoalkane gains hydrogen, the product being a salt.
C2H5NH2 + HCl ==> C2H5NH3+ + Cl-
(2) Ligands: Both aminomethane and aminoethane behave like ammonia and form complex ions with transition metals. Taking aminoethane and copper, the blue complex ion tetraaminoethanecopper (II) is formed:
4 C2H5NH2 (aq) + Cu2+(aq) ==> [Cu(C2H5NH2)4]2+(aq)
(3) Nucleophilic Substitutions: As the aminoalkanes have a lone pair of electrons, and a strong polar bond between the nitrogen and hydrogen of the amine group, they are strong nucleophiles, drawn to positively charged groups such as the carbon of a haloalkane. When aminoethane is added to a haloalkane e.g. chloroethane, it forms a secondary amine:
CH3CH2NH2 + CH3CH2Cl ==> CH3CH2NHCH2CH3 + HCl
The HCl then goes on to react with the primary amine, forming ethylammonium chloride:
CH3CH2NH2 + HCl ==> CH3CH2NH3+ + Cl-
Overall, the reaction equation is thus:
2CH3CH2NH2 + CH3CH2Cl ==> CH3CH2NHCH2CH3 + CH3CH2NH3Cl
|

