|
Alkenes are also a homologous series of hydrocarbons. Unlike the alkanes, they contain a C=C double bond, and are therefore unsaturated. This double bond is known as a functional group (a specific arrangement of atoms and or bonds responsible for the main properties, chemical and physical, of an organic molecule). Despite this difference, solubility, melting point and boiling point values, and conductivity are all very similar to those of alkanes.
| In naming alkenes, the parent chain keeps the same prefixes: meth- eth- prop- and so forth. The suffix is –ene, with a number separating the prefix and suffix to indicate the carbon atom where the double bond starts e.g. pent-2-ene.
|
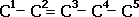
pent-1-ene |
| The carbons are numbered to give the lowest number to the double bond, and side chains are numbered accordingly e.g. 3-methylpent-1-ene.
|
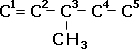
3-methylpent-1-ene |
Like alkanes, structural isomerism occurs, either due to side chains, and their positions, or because of variation in the position of the double bond. Cycloalkanes are also isomeric. Apart from the regular structural isomers, alkenes can also form another type of isomer: the geometric isomer. Geometric isomers arise as carbon atoms attached to the double bond cannot rotate. Therefore, the groups attached to these carbons are also fixed in their positions.
As an example, we shall take but-2-ene. Arrangement [1] is different from arrangement [2]. In arrangement [1], the same groups are on the "same" sides of the double bond. This geometric isomer is the "cis" isomer: cisbut-2-ene. Arrangement [2] shows the "trans" isomer, where the like groups are on opposite sides of the molecule: transbut-2-ene.
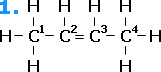
cisbut-2-ene |
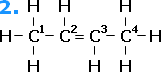
transbut-2-ene |
Alkenes can readily react with many small molecules, by adding them across the double bond. These are called addition reactions. Several such important reactions are examined below.
(1) Hydrogenation reactions involve the addition of hydrogen across the double bonds. In the case of ethene, this reaction requires a catalyst of nickel, palladium or platinum, to a temperature of 150 to 200°C and under 4 atmospheric pressures (about 400kPa). The result is ethane -
C2H4 + H2 ==> C2H6
This reaction is important in the production of margarine. Plant oils, which are polyunsaturated, are hydrogenated to reduce the number of double bonds to such an extent that the oil solidifies.
(2) Bromination reactions are the usual test to identify alkenes. Bromine is added in solution, and bonds to the carbons across the double bond, forming a bromoalkane (alkane as all bonds are now saturated). As bromine is used up from solution, it changes colour from pale brown to colourless. The reaction equation for ethene is:
C2H4 + Br2 ==> CH2Br-CH2Br
This decolourisation is rapid, and is thus good as an indicator of alkenes. Alkanes react slowly, even in direct sunlight.
(3) Hydration reactions involve the addition of water to an alkane, resulting in the formation of an alcohol.
C2H4 + H2O ==> C2H5OH
The water molecule is broken into an H group and an OH group, which then attach to the double bonded carbons. The process requires a dilute sulfuric or phosphoric acid catalyst at 330°C and 60 atmospheric pressures.
(4) Just as halogens can be added across the double bond, so too can hydrogen halides. The hydrogen and halide groups will bond to each of the double-bonded carbons to form a haloalkane.
(5) Ethene is readily combustible, and combustion products depend on the air supply. Energy and water are also released, as well as a mixture of carbon dioxide and monoxide gases. (CO2 will be produced if there is sufficient air available)
(6) Polymerisation (in this case addition polymerisation) involves the joining of many monomers together to form one giant molecule. In the case of ethene, the double bond splits, and each ethene molecule will bond to two others forming a long chain.
Unsymmetrical alkenes
| An unsymmetrical alkene has different groups attached to the two carbons involved in the double bond.
| 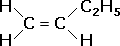
but-1-ene |
When dealing with addition reactions of unsymmetrical alkenes, Markovnikov's rule must be applied to determine the major product of the reaction.
The hydrogen atom from water or a hydrogen halide will bond to the carbon that has the most hydrogens already attached (The rich get richer). For example, when adding water to but-1-ene, the hydrogen group will bond to the first carbon, and the hydroxyl group will attach to the second carbon atom, forming butan-2-ol.
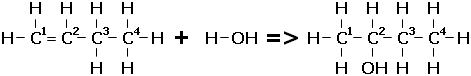 Small quantities may also form of the other possible product - the minor product. In this case, it would be butan-1-ol. Small quantities may also form of the other possible product - the minor product. In this case, it would be butan-1-ol.
The Markovnikov rule can also be used in regards to dehydration reactions in order to determine the major reaction product. For, say, butan-2-ol, the hydrogen could be lost from either of two carbons (C1 and C3 in the reaction diagram below). In most cases, the major product will be but-2-ene, the hydrogen being removed from the carbon with the least hydrogens.
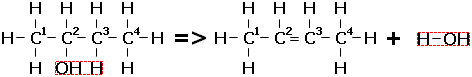 Markovnikov's rule can be summarised thus – "In addition reactions, the most hydrogen rich carbon gets 'richer' by gaining hydrogen. In elimination reactions, the 'poor' carbon gets 'poorer', losing hydrogens".
Markovnikov's rule can be summarised thus – "In addition reactions, the most hydrogen rich carbon gets 'richer' by gaining hydrogen. In elimination reactions, the 'poor' carbon gets 'poorer', losing hydrogens".
|

